Simulation of Cardiac Devices & Drugs for in silico Testing and Certification
Call H2020-SC1-DTH-2018-2020, Research and Innovation Action (Topic SC1-DTH-06-2020)
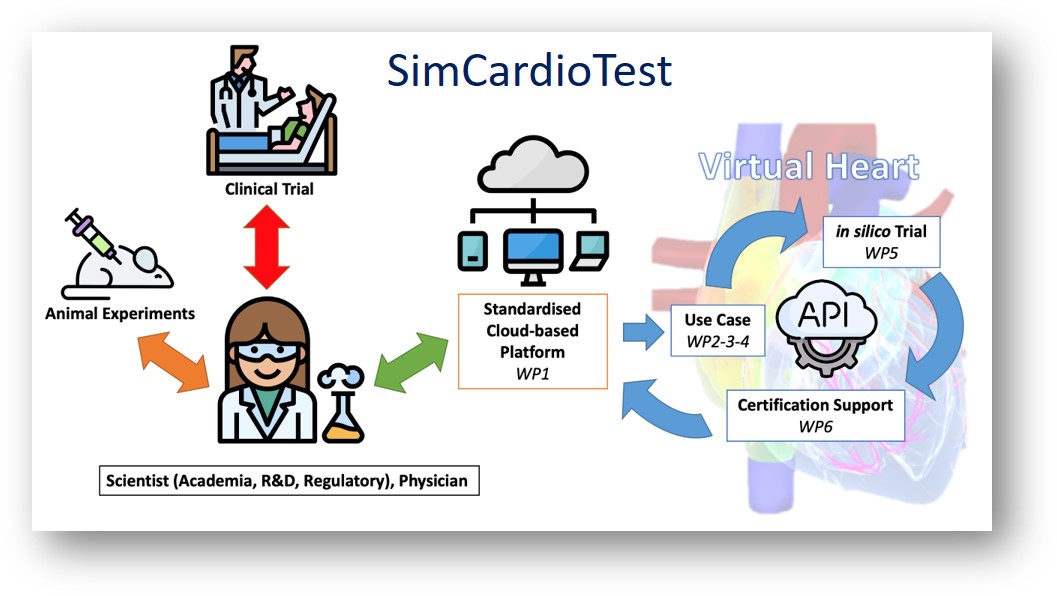
Despite massive investment in healthcare, huge R&D cost increase and regulatory pathway complexity hamper tremendously commercialisation of new devices & medicines, putting patient populations at risk of not receiving adequate therapy. At the same time, outside healthcare, computer modelling and simulation (CM&S) is precisely recognised to increase speed & agility while reducing costs of development. CM&S can create scientific evidence based on controlled investigations including variability, uncertainty quantification, and satisfying demands for safety, efficacy & improved access. Cardiac modelling has dramatically gained maturity over the last decades, with personalisation to clinical data enabling validation. We selected a number of cardiac devices and medicines where CM&S is mature enough and that represent the most common cardiac pathologies, to demonstrate a standardised and rigorous approach for in-silico clinical trials. SimCardioTest will bring a disruptive innovation by creating an integrated and secure platform standardising & bridging model simulations, in-silico trials, and certification support. This environment will go beyond the state-of-the-art in computational multi-physics & multi-scale personalised cardiac models. Diseased conditions and gender/age differences will be considered to overcome clinical trials limitations such as under-representation of groups (e.g. women, children, low socioeconomic status). Advanced big data, visual analytics & artificial intelligence tools will extract the most relevant information. It is critical that Europe demonstrates its capacity to leverage in-silico technology in order to be competitive in healthcare innovation. SimCardioTest exploitation aims at delivering a major economic impact on the European pharmaceutical and cardiac devices industry. It will accelerate development, certification and commercialisation, and will produce a strong societal impact contributing to personalised healthcare.
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 101016496
The available information is expanded on the project website:
Under execution.